What Term Best Describes the Fluorine Atom in This Reaction
Reaction 1 CH3CHBrCH3 NaCN NaBr reaction 2 CH3CHBrCH3 Nal CH3CHICH3. Given the equation representing a reaction.
Fluorine Chemical Element Reaction Water Uses Elements Examples Metal Gas Number
Min is asked to balance the equation below.
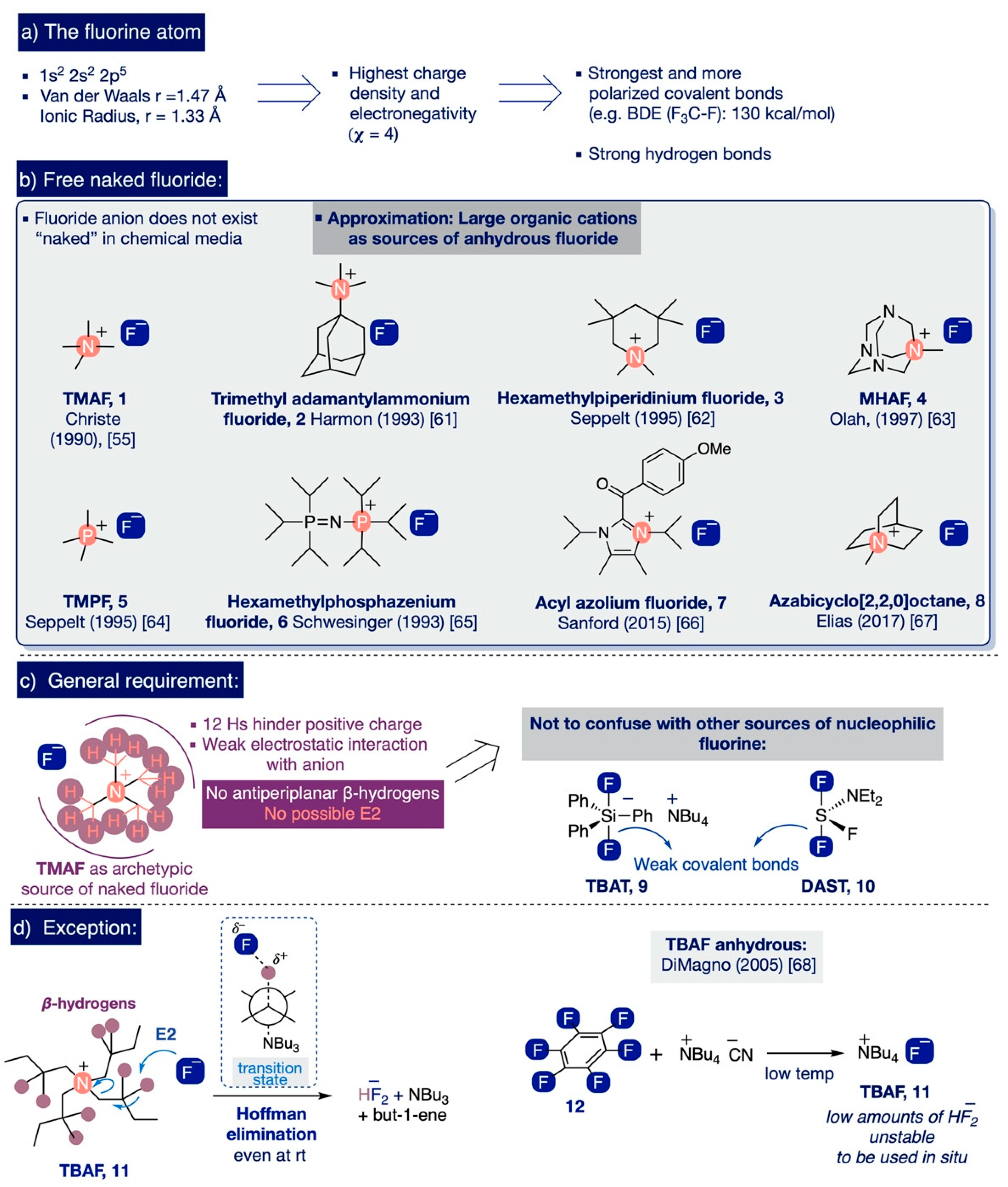
. An element from the sodium is shared with the fluorine atom forming fluoride iron. The metal can take on any of its valences. Describe the behaviour of the metal in the reaction.
The CF bond is stronger than the CCI bond. Which term indicates how strongly an atom attracts the electrons in a chemical bond. 00 non -polar c.
Tes 3 F2gTeF6s Reaction of fluorine with noble gasses. Fluorine 5 Which group on the Periodic Table has two elements that exist as gases at STP. Identify the polymeric material that is produced from this reaction.
In a nonpolar covalent bond electrons are. The compound formed when Fluorine and Sodium reacts Sodium Fluoride is ionic. 1 carbon 2nitrogen 3oxygen 4 fluorine.
The reaction between magnesium metal and oxygen to form magnesium oxide involves the oxidation of magnesium. View Question 1png from ANATOMY 169 at Rowan-Cabarrus Community College. Atom is the smallest component of an element having the chemical properties of element containing of nucleus that contains neutrons and protons electrons are bound to the nucleus by electrical attraction.
1 Group 1 3 Group 16. 15 polar 7____A multivalent metal is involved in a synthesis reaction. A bond is formed and energy is absorbed.
Atom is the basic unit of matter. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H. The atom having the valence-shell configuration 4s 2 4p 5 would be in.
Select the term best describing the series of elements. In the work 17 fluorine atoms were produced by the dissociation of 985 pure F 2 gas using 14 MHz discharge in an alumina tube 50 cm upstream of the reaction cell. Protons neutrons and electrons.
Which would be an ionic bond that could form from any of these ions. Atom is made up of three parts. Fluorine has one electron space available to form a bond What is the total number of electrons present in the Lewis dot structure of an individual hydrogen atom H.
When an atom of sodium and an atom of fluorine combine to form the salt sodium fluoride a bond is formed. Why are there and - signs on the elements after the electron is transferred. HELP ASAP 2NH3 OCl N2H4 H2O Cl A Reducing agent B Oxidized C Reduced D Electrolyte im thinking reduced maybe but not sure.
15 non -polar b. As the most electronegative element it is extremely reactive as it reacts with all other elements except for argon neon and helium. This diagram represents a neutral atom of fluorine-19.
Share electrons equally with other elements. Which best describes the reaction. 3 question At about 302 an atom of Lithium loses an electron to an atom of Fluorine.
Which term is used to describe the attraction that an oxygen atom has for the electrons in a chemical bond. Which type of bond exists between an atom of carbon and an atom of fluorine. The C-F bond is the weakest bond between carbon atom and a halogen.
Pull electrons away from other elements. Which statement best describes the reaction. The chlorine atom is larger than the fluorine atom.
Fluorine radicals are less stable than chlorine radicals. Its monatomic form H is the most abundant chemical substance in the Universe constituting. Between carbon and fluorine.
Mn Fe Co Ni Cu. A What chemical equation best describes this reaction. Under identical conditions even though it proceeds by the same mechanism reaction 1 is faster than reaction 2.
A Group VIA and Period 5 b Group IVB and Period 4 c Group VIB and Period 7 d Group VIIA and Period 4 e Group VIIB and Period 4 2. Give electrons away to other elements. What do mean by oxidize.
O O O2 Which statement describes the changes. Which term describes the following mechanistic step in the catalytic cycle of the Heck reaction. Is this a chemical reaction.
Which term best describes all atoms in ionic bonds. With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. In a particular redox reaction MnO2 is oxidized to MnO4 and Ag is reduced to Ag.
2 An atom that contains six protons six neutrons and six electrons has a mass of approximately 1 12 u 3 18 u. Which statement best describes the Lewis dot structure for fluorine. The term oxidation was originally used to describe reactions in which an element combines with oxygen.
A d-transition metals b representative elements c. Forming Bonds Quick Check 3 of 53 of 5 Items Question Fluorine is the most electronegative element on the periodic table which means that in an ionic bond fluorine will always 1 point share electrons unequally with other elements. The reaction of 500 g fluorine with excess chlorine produced 560g chlorine trifluoride in the laboratory.
Fluorine is a chemical element with the symbol F and atomic number 9. Ionic compounds are formed when a non-metal such as Fluorine reacts with a. Which type of bond exists between an atom of carbon and an atom of fluorine.
Krypton will react with fluorine F2 when cooled to -196 C liquid nitrogen and zapped with an electric discharge or X-rays forming kryptonII fluoride KrF23. An example of a polymerization reaction is the reaction of a glycol with an organic diisocyanate. 1 calcium Ca 2 fluorine F -1 and sulfur S -2.
Which term best describes Cl in the following reaction. Which statement correctly describes the behavior of valence electrons in this bond. Which atom has the least attraction for the electrons in a bond between that atom and an atom of hydrogen.
Tellurium reacts with excess fluorine forming telluriumVIfluoride 3. Why or why not. It is the lightest halogen and exists at standard conditions as a highly toxic pale yellow diatomic gas.
In the course of this reaction each magnesium atom loses two electrons to form an Mg 2 ion. Complete and balance the equation for this reaction in acidic solution.
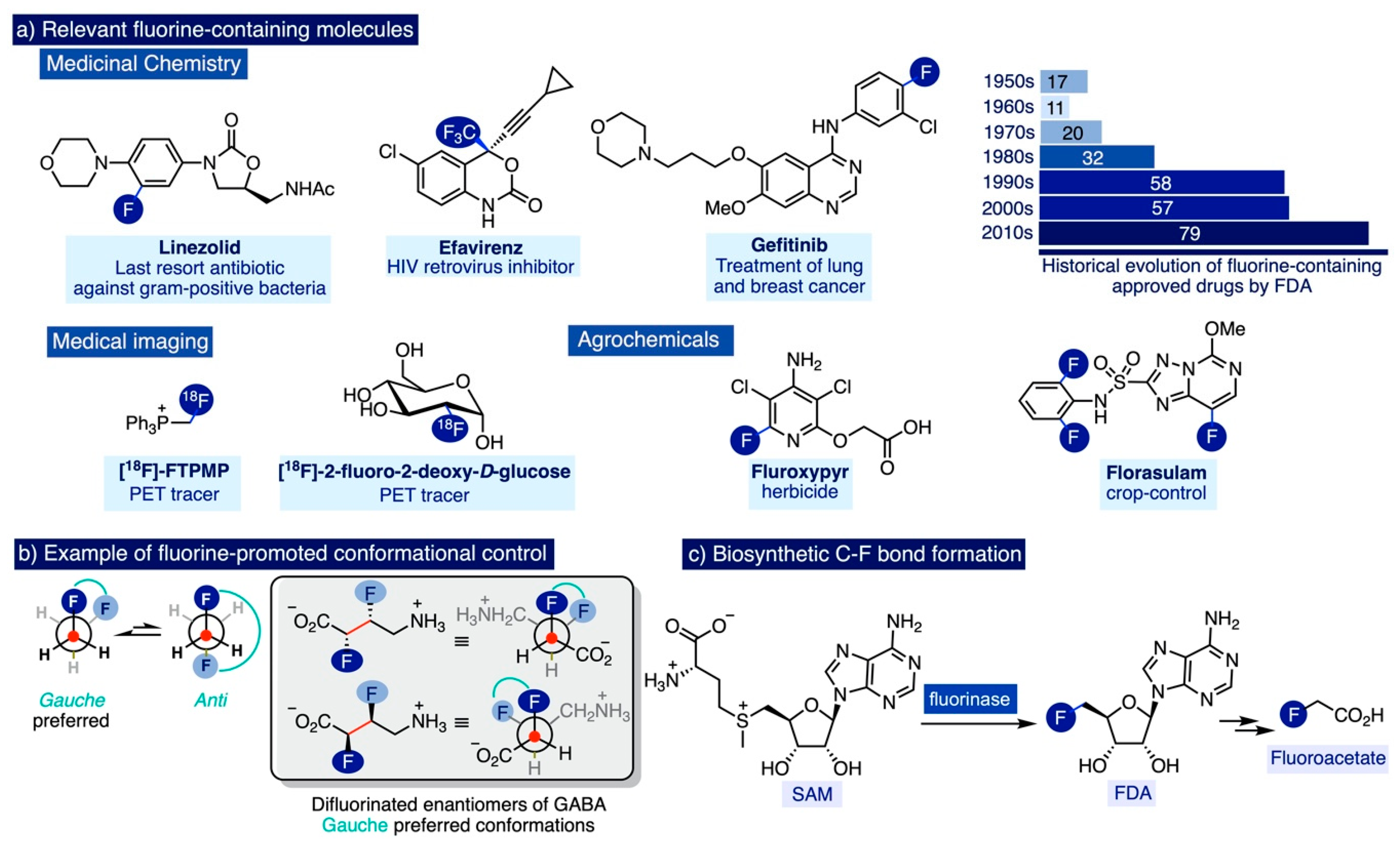
Catalysts Free Full Text Tetramethylammonium Fluoride Fundamental Properties And Applications In C F Bond Forming Reactions And As A Base Html

Catalysts Free Full Text Tetramethylammonium Fluoride Fundamental Properties And Applications In C F Bond Forming Reactions And As A Base Html

No comments for "What Term Best Describes the Fluorine Atom in This Reaction"
Post a Comment